Abstract
Background: Disease relapse has emerged as the most common cause of treatment failure after transplantation. Natural killer (NK) cells have potent antitumor and antiviral effects. We hypothesized that multiple infusions of ex vivo expanded NK cells administered in early post-transplant will enhance graft-versus-leukemia (GVL) effect and began a phase 1/2 clinical trial in haploidentical transplant patients (clinicaltrials.gov NCT01904136).
Methods: Results from the phase 1 clinical trial were recently reported. The aim was to determine the maximum tolerated dose of ex vivo expanded NK cells with a K562 expressing mb-IL21 feeder cell system in patients with myeloid malignancies (AML/MDs/CML). Patients received conditioning with melphalan 140mg/m2, fludarabine 160mg/m2 and 2GyTBI, and GVHD prophylaxis with post-transplant cyclophosphamide, tacrolimus and mycophenolate. All patients had bone marrow graft. NK cells were generated from peripheral blood mononuclear cells of the same donor and infused fresh on Day -2, and cryopreserved on Day +7 and +28 (up to 90). Having an NK-cell alloreactive donor or certain KIR genotype was not a requirement to participate in this study, although these characteristics were evaluated in all patients. In the phase 1 study dose escalation patients received NK cells starting at 1x105 to 1x108/Kg/dose for 3 doses. No dose limiting toxicities were observed in the phase 1 study. 1x108/kg/dose was chosen for the phase 2 trial.
Results: Twenty patients were treated to date, 13 on the phase 1 study and 7 on the phase 2 part. The median age was 45 years (range 18-59), the median follow-up survivors was 16.5 months. Twelve patients (60%) were females. The median HCT-CI was 2 (range 0-8). Ten patients (50%) had AML (9 in CR1 and 1 beyond CR2), 3 (15%) had MDS (2 with induction failure, 1 untreated), and 7 (35%) had advanced CML (4 in CP2 after progression to blast-phase of which 2 in CNS, 1 in CP1, 1 clonal evolution). Of the patients with AML 4 patients with AML had poor-risk cytogenetics and 6 had intermediate with high-risk mutations except one (2 had FLT3, 2 had NRAS, 1 had ASXL1) and 3 patients had primary induction failure. Two MDS patients had complex cytogenetics and one diploid with high percent of bone marrow blasts. DRI was very high/high in 10 patients and intermediate/low in 10 patients. All patients received all 3 NK cell infusions as planned except one patient. Eight patients received the maximum dose of 1x108/kg/dose.
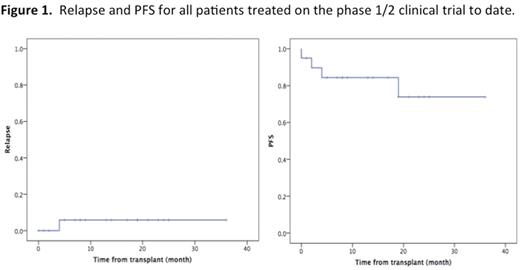
One patient had early death due to hemorrhage. Nineteen patients were evaluable for engraftment and all achieve primary engraftment after a median time of 19 days (range 16-42). Nine of 12 female patients (75%) had donor-specific anti-HLA antibodies. Chimerism was 100% donor in all patients except one who had mixed chimerism. The cumulative incidence (CI) of all aGVHD at Day100 and 1 year post-transplant was 26.1% and 48% (3 patients had late acute GVHD), exclusively grade 2 and exclusively GI tract. No grade 3 or grade 4 aGVHD, and no chronic GVHD was observed. Also, none of the patients developed liver GVHD. The CI of TRM at 1 and 2 years post-transplant was 5.6% and 16.7%, respectively. Only one patient relapsed to date for a CI of relapse at 1 and 2 years on 5.6%. The only patient who relapsed was an advanced FLT3+AML patient (beyond CR2) who received the lowest NK cells dose. None of the patients who received ≥1x105/kg dose relapsed. The median overall survival and progression-free survival (PFS) were not reached. The 1-year and 2-years PFS was 84.4% and 74%, respectively (Figure 1). Eight of 19 patients (42%) reactivated CMV and 4 patients (21%) had grade 1 transient BKV cystitis.
Conclusions: Multiple infusions of high doses of donor-derived ex vivo expanded NK cells with a K562 feeder cell system and mbIL-21 administered early post-transplant are safe and are associated with low relapse rate and low incidence of viral reactivation. A randomized trial is needed to confirm these results.
Oran: Celgene: Research Funding; AROG: Research Funding; Astex: Research Funding. Lee: Courier Therapeutics, Inc: Membership on an entity's Board of Directors or advisory committees; Cyto-Sen Therapeutics, Inc: Other: Founder, Vice President, Medical Director; Intellia Therapeutics, Inc: Consultancy; Miltenyi: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal